Worse than MRSA: Doctors call for urgent action on deadly superbug threat
Doctors are warning that the rise of an almost untreatable superbug, immune to some of the last-line antibiotics available to hospitals, poses a grave threat and needs urgent government action.
The Bureau has obtained data showing 60 people treated at Central Manchester University Hospitals NHS Foundation Trust (CMFT) since 2009 have died after suffering a bloodstream infection from carbapenemase-producing enterobacteriaceae (CPE) - dubbed the "nightmare” bacteria by infection experts - four times as many as previously reported.
Reports of CPE bacteria - which kill 40 to 50% of all people who get a bloodstream infection - have risen more than five-fold across England in the last five years. It is more dangerous than MRSA, yet unlike MRSA it is not mandatory for NHS trusts to report CPE cases to Public Health England (PHE). PHE admits it doesn’t know where the CPE infections are coming from or how many people are dying.
Poorly-designed sinks which allow contaminated water to splash back out of drains are thought to have played a major role in the Manchester outbreak. The same types of fittings are common in hospitals throughout the country.
In London there have been ten occasions in which a patient has caught CPE because the last person to sleep in their hospital bed was carrying it.
“Shocking” figures, previously unrevealed
Freedom of Information requests made by the Bureau reveal at least 81 people infected with CPE have died since 2009 at 66 NHS trusts in England (though CPE may not have been the direct cause of death in all cases). The real figure is almost certain to be much higher because many trusts did not respond to our requests or were unable to supply complete data.
Alongside Manchester and London - where dealing with CPE has cost NHS trusts almost £10 million - there have been confirmed outbreaks in Liverpool, Leeds, Sheffield, Birmingham, Nottingham, Colchester, Edinburgh, Belfast, Dublin and Limerick, among others.
Samples of CPE bacteria sent to Public Health England by trusts for testing rose from three in 2003 to almost 2,000 in 2015.
The numbers revealed by the Bureau were “shocking,” said Val Edwards-Jones, emeritus professor of microbiology at Manchester Metropolitan University. “It should absolutely be mandatory for trusts to report this,” she said. “If you go back to the 1990s MRSA [reporting] wasn’t mandatory. It was only when hospitals did proper surveillance and began looking at the bugs in the blood that we knew the scale of the problem. Then it was found that there were certain things that weren’t being done correctly.”
Shadow Health Secretary Jonathan Ashworth said he would call for an immediate response by the government to the Bureau’s findings.
“Frankly it beggars belief that it is not mandatory to report cases of CPE bloodstream infections to Public Health England, as it is with MRSA and C Difficile,” he said. “Serious questions must now be asked of the Government’s incompetence in tackling AMR and we urgently need a review into CPE.”
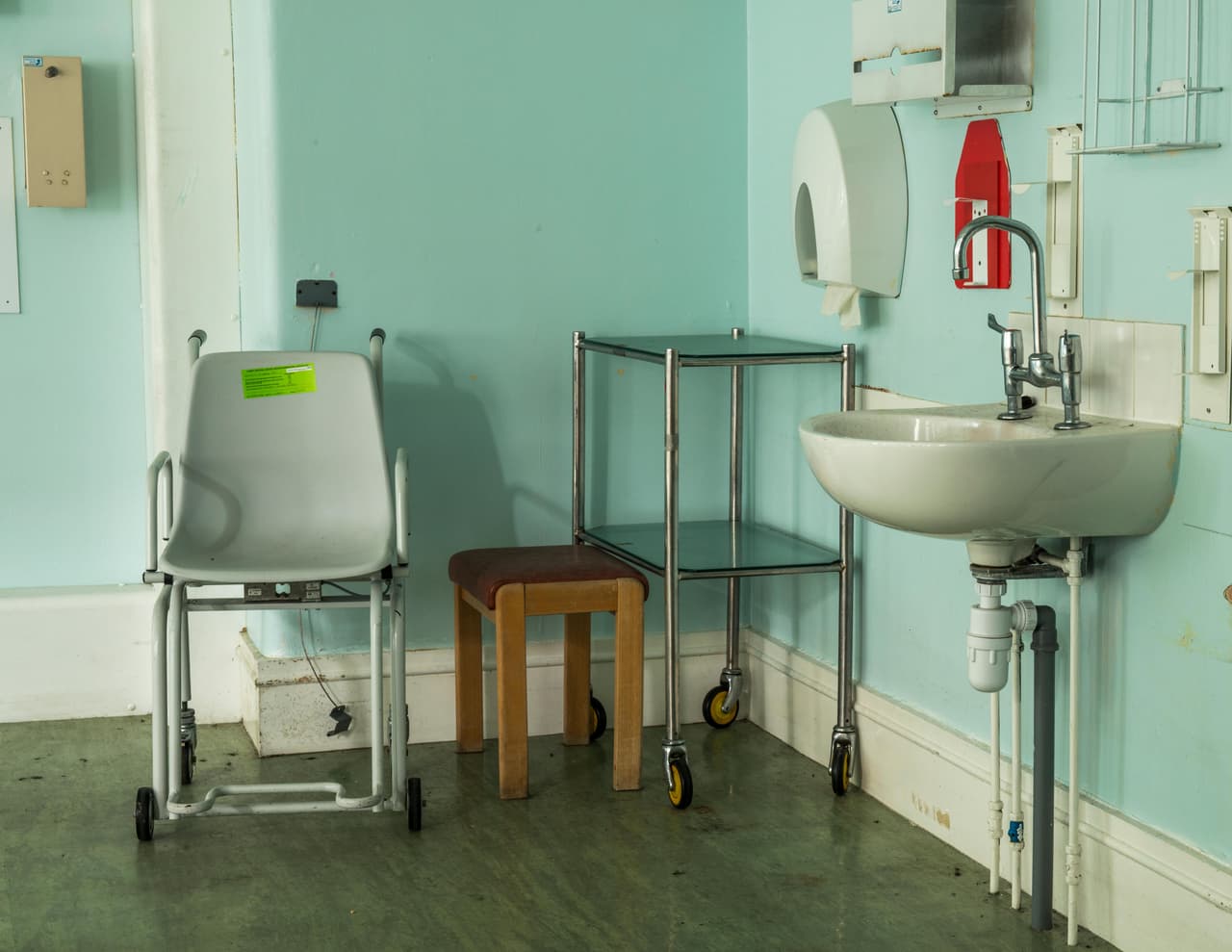
How badly-designed sinks and basins spread the bugs
In 2015 the continued investigation into the Manchester outbreak discovered that CPE bacteria were living in the kitchen sinks and handwash basins, and that the types of drains used in the basins allowed water to splash back up and spread the infection to patients. New drains fitted with siphons that stopped splashback were installed in Manchester Royal Infirmary’s heart centre, where the latest cases of the bug had been found - but the old sink and drain designs are still used in other wards, and are commonplace in hospitals across the country.
Hospitals should be checking their sinks and drains for superbugs and all sinks should be replaced, said Hugh Pennington, a leading microbiologist who chaired official inquiries into E.coli outbreaks in 1996 and 2005. It shouldn’t take an outbreak for hospitals to think about how the designs of sinks and handwash basins could be causing bacteria to spread, he added.
“It goes back to basics,” he said. “These bugs shouldn’t be in the sinks in the first place, particularly in areas where there are vulnerable patients.
“We know sinks have been a problem in the past. We’ve had pseudomonas outbreaks in neonatal units where the environmental sources [which include sinks] seem to be important. It’s all about sound plumbing. It's not rocket science. If your sink is going to spread the bug, get rid of it.”
Urgent action required
It is imperative that the NHS and PHE take preventative action to stop deadly superbugs spreading, instead of reacting to outbreaks when they happen, say microbiologists across the country. The only way they can do this properly is if reporting of CPE infections and related deaths is made mandatory, they argue.
If the UK does not do this, it is inevitable that the lethal bug will spread and many more people will die, they warn. Italy is held up as an example - it went from having sporadic cases of CPE in 2009 to the bugs being rife across the country by 2014, due to a lack of good surveillance and infection control.
This has had severe implications, said Dr Matthew Laundy, consultant medical microbiologist at St George’s University Hospitals NHS Foundation Trust.
“If you look at Italy they’ve suspended bone marrow transplant programmes,” he said. “If you’ve got no antibiotics to treat CPEs you’re stuck.”
CPE is worse than MRSA, Landy stressed. “MRSA was never pan-resistant - there were other drugs we could use. It was never as though there were no options. With CPE, we’re down to one option, or no options.”
CPE rates should be made public and published on wards so patients know which hospitals are affected, said Dr Michael Millar, a consultant microbiologist at Barts Health NHS Trust. This would motivate managers to address problems in infection control, he said.
“Nightmare bacteria”
Carbapenemase-producing enterobacteriaceae - CPE - refers to a group of bacteria which have acquired the ability to produce enzymes that fight off whole classes of antibiotics. They are given that ability by various genes which have developed as the phenomenon of antibiotic resistance has grown.
The CPE genes even give immunity to carbapenems – a group of ‘last resort’ antibiotics that are used on serious infections resistant to all other drugs. Some variants of common infections such as E.Coli and Klebsiella have now acquired the genes.
CPE bacteria can live in the gut without causing any harm but can lead to serious infections if they get into wounds, lungs, urine or the bloodstream. Part of a family that includes bugs known as CRE or CPO, they have been dubbed the “nightmare” bacteria by America’s Center for Disease Control and Prevention because they are so lethal.
If CPE bacteria get into the blood, studies show between 40% and 50% of patients die. A CPE infection can still be treated but combinations of antibiotics or older, more toxic drugs have to be used as doctors have no other choice. It is a problem in hospitals and nursing homes where patients might have weak immune systems or be fitted with tubes which can let bacteria into the body.
There is a high prevalence of CPE in India, Bangladesh, Pakistan, the Middle East, south and Central America, China, southeast Asia, Taiwan, Japan, some countries in southern Europe and the USA. In February the World Health Organisation named carbapenem resistant bugs a ‘critical priority’ for which new antibiotics are urgently needed.
Antibiotic resistance, also known as antimicrobial resistance (AMR), is considered one of the gravest public health threats facing the world. It has been estimated that it’s already killing hundreds of thousands of people a year worldwide, but a lack of reliable data means there is no way of knowing the true figure. However we do know that sepsis caused by drug-resistant infections is killing more than 56,000 newborns in India and nearly 26,000 newborns in Pakistan each year, just two examples of the scale of the problem.
Full scale of the problem is unknown
The Bureau submitted FOI requests to all 136 NHS trusts in England requesting figures on CPE infections and deaths between 2009 and 2016.
In total, 97 responded, though the types of response and data they were able to provide varied greatly. Nearly half did not keep records on CPE infections or could not extract them; 12 trusts did record infections but did not record deaths; and of those that recorded both infections and deaths, the time periods the data covered was different. (Inconsistent or non-existent data collection is typical when it comes to AMR, despite the huge threat to public health posed by such infections. Throughout the UK and the world there is no standardised systems for recording AMR infections or deaths, meaning available data is very poor, as previously highlighted by the Bureau.)
What we can see from the data is that at least 81 people died over the time period at 67 trusts - though CPE may not have caused all the deaths. The real figure is likely to be much higher.
When a hospital does not have facilities for molecular diagnostics and it suspects it may have a patient carrying a certain superbug, it sends samples of the bacteria to PHE’s laboratory. In 2003 PHE received just three samples of CPE bacteria. By 2015 this had risen to 1,893.
“Whatever is being sent to Public Health England, you can double that, because screening will only pick up half of cases,” said Dr Michael Weinbren, a consultant in infectious diseases and infection prevention and control at Chesterfield Royal Hospital NHS Foundation Trust.
The Bureau also discovered another 21 people died after a CPE outbreak at Imperial College NHS Foundation Trust - the figure was revealed at a workshop for doctors. A trust spokesman acknowledged the patients did have CPE, but said an internal review had concluded it was not the direct cause of death in any of the cases.
Our data and information from other sources shows London is the biggest CPE hotspot after the northwest, where outbreaks were recorded at nearly all hospital trusts. Smaller outbreaks have occurred at trusts throughout the UK, with hospitals forced to close wards.
In London, a 10-month outbreak across Imperial College Healthcare Trust cost £1 million, covering a whole raft of expenses from deep cleaning and ward closures to special training for nurses and expensive drugs. It is estimated the outbreak in Manchester cost £8.4 million.
PHE told the Bureau it wants to improve how it monitors CPE. “We are working closely with the NHS to further improve data collection and surveillance and have also published advice to help hospitals in detecting, managing and controlling infections caused by CPE,” said Professor Alan Johnson, head of the Department of Healthcare Associated Infection and Antibiotic Resistance.
He stressed that less than 2% of E Coli or Klebsiella bloodstream infections are resistant to carbapenems, and that CPE infections could be treated with combinations of antibiotics (though as microbiologists explained to the Bureau, this only works in some cases. Around half of all people who get a bloodstream infection die).
The Manchester outbreak
Manchester has had an ongoing problem since 2009 with CPE – mainly a type called KPC (Klebsiella pneumoniae carbapenemase ). In 2014 it confirmed 14 people with a bloodstream infection had died in the past four years.
However our research shows the outbreak was far more serious. In fact 61 people have died in seven years, including a six-year-old boy with leukaemia who caught the infection while undergoing a bone marrow transplant.
Many of those affected were very ill and had underlying medical problems and so it is not known if the infection directly caused their death. Some may have died with a CPE infection rather than from it.
Within a year of the first CPE case being found in Manchester Royal Infirmary in 2009, 185 people were found to be carrying the bug. Six people developed bloodstream infections, three people were admitted with infections, and four people died. The outbreak peaked in 2014, with 783 people carrying the bug, 17 people developing bloodstream infections and 14 deaths. Since then, there have been fewer cases.
CPE also spread to other hospitals in the Greater Manchester area, and the outbreak in the northwest also spread to Liverpool, where there have been hundreds of reports of the bug.
AMR: A global public health threat
Read more from the Bureau's antibiotic resistance teamIn 2013 the Central Manchester NHS trust brought in sophisticated testing so it could diagnose CPE bacterial infections within 24 hours, alongside screening of every patient being admitted to hospital. If patients had travelled abroad in the past year or been in a UK hospital in the UK known to be affected by CPE, they are deemed high risk, put in a side room or on a special ward and given a rectal swab.
The screening is far from fail-safe - it only picks up around half of people who are carrying the bug.
Despite the precautions taken in Manchester, in 2015 the bug was found in the Heart Centre in the Manchester Royal Infirmary building. Two wards had to be closed on four occasions for deep cleaning, but the usual infection control measures didn’t work and the bug continued to be found.
It was at this point it was discovered bacteria were living in the kitchen utility sink, drains and handwash basins. As part of a 13-week refurbishment, the drains were replaced and siphons added to the sinks and handwash basins, to ensure the water flowed down the drain and didn’t splash back up.
New cleaning methods were used on all sinks and handwash basins, and the wards and hospital rooms were swabbed regularly for CPE. The trust said these measures successfully controlled the outbreak.
"Infection prevention and control will always be a high priority for the Trust and is central to our patient safety agenda," said Cheryl Lenney, Chief Nurse at CMFT. "We are continuing our efforts to control the spread of CPE and all other organisms."
Surveillance urgently needed
Some experts believe all patients should be screened for CPE when they come into hospital, as was the case for MRSA until 2014.
But screening and overall surveillance of CPE bacteria and infections is still poor. Susan Hopkins, an epidemiologist and consultant in infectious diseases at Public Health England told a recent conference in London that the organisation had tried to develop a better system for tracking CPE but the data was “pretty rubbish”. Dr Hopkins told delegates: “If someone says to me ‘Where are the carbapenemase cases coming from, are they coming from overseas, are they coming from outbreaks?’, I say “I don’t know’”.
For patients found to be carrying the bug, there is currently no way of getting rid of it from the gut. CPE-positive people can be stigmatised. Doctors have told the Bureau it can be difficult to get nursing home places for patients with CPE. They have witnessed families turning away relatives with the bug for fear they would catch it.
The lack of data on CPE is a huge problem, says Dr Michael Cooper, a consultant microbiologist and director of infection control at the Royal Wolverhampton NHS Trust.
‘“If something’s not mandatory, it’s the places doing well that take care to report,” he said. “You don’t get figures from the trusts with their head in the sand, the poor performers. To extrapolate from this is dangerous.
“Public Health England have no idea how many people are dying, they’ve no outcome data. This is a serious mistake.”
Main image of Klebsiella Pneumoniae (computer illustration) by Alamy
Background image of hospital ward by Simon Webster/Alamy


